Download this article as a PDF
Introduction
The formation of iron oxide – known as rust – is a persistent and costly problem across numerous industries, from automotive and construction to maritime and household applications, as well as all kinds of everyday objects. Caused by the oxidation of iron and its alloys, rust weakens structures, reduces equipment longevity, and diminishes aesthetic appeal. Beyond cosmetic concerns and property damage, rust poses serious safety risks by causing machinery and other production equipment to malfunction. Thus effective rust removal is essential to maintain and restore metal components.
This widespread issue has created a robust market for chemical rust remover products designed to eliminate corrosion. These products are typically applied as soaking solutions, sprays or gels. Most conventional rust removers are based on acids, such as phosphoric, hydrochloric and sulfuric acid, or chelating agents like ethylenediamintetraacetic acid (EDTA). These substances work by dissolving rust and converting iron oxide into a soluble form. Their popularity stems from their speed and effectiveness in removing corrosion across industrial and domestic settings.
However, despite their efficacy, these chemicals raise significant environmental and health concerns: exposure to fumes of strong acids like hydrochloric acid or accidental skin contact can cause burns, inhalation injuries, and eye injuries. Improper disposal of these chemicals may lead to water pollution or soil contamination. Additionally, strong acids can dissolve paint, damage finishes and even corrode the base metal itself.
With increasing awareness of health and environmental issues, the demand is growing for safer, more eco-friendly solutions. Both industry and consumers are shifting towards modern formulations with plant-derived ingredients. Organic acids such as citric acid address these concerns, offering comparable performance but with significantly lower toxicity. This is not only true for active ingredients, but also for additional ingredients like thickeners, for example in gel applications. In addition to consumer expectations, stricter environmental regulations around hazardous chemicals are accelerating the development of safer and more eco-friendly consumer products. Thus the emergence of bio-based and sustainable rust removers presents a promising opportunity for the market, aligning with global sustainability trends and consumer preferences for safer, greener solutions.
Eco-friendly and safe ingredients for rust removal solutions
Citric acid is a naturally occurring acid, manufactured by fermentation of renewable raw materials such as carbohydrates from corn. It is a generally permitted food additive (E 330) in Europe and is affirmed as generally recognised as safe (GRAS) by the US Food and Drug Administration. With its non-hazardous handling and favourable ecological profile, citric acid has proven to be a viable bio-based alternative to commonly used mineral acids. The risk to both humans and the environment is minimised since citric acid is a non-toxic ingredient. It does not emit harsh chemical fumes and is generally safe to handle. Additionally, citric acid is readily biodegradable and can be disposed of with regular sewage or waste. Its low aquatic toxicity makes it less harmful to water systems than phosphates or synthetic chelating agents like EDTA. Easy-to-use gel formulations of rust removers, which are helpful for vertical surfaces and detailed spot treatment, require an acid-stable thickener: xanthan gum, a polysaccharide found in nature, is readily biodegradable and represents an optimal solution for this application. Fermentation-based manufacturing processes using renewable raw materials, combined with safer handling characteristics, make these Jungbunzlauer ingredients a compelling choice for acid-based rust removal solutions, especially in household and low-risk industrial settings. In addition, both citric acid and xanthan gum (acid-stable grade) are ECOCERT approved raw materials of 100% natural origin for use in detergents.
Comparative testing of citric acid vs phosphoric acid
The investigation focused on comparing the widely used industrial phosphoric acid (PA) with the more sustainable alternative, citric acid (CA), for treating oxidised steel substrates.
The objective was to evaluate the derusting performance of both acids under controlled conditions and to examine the stability of different formulations. Cold-rolled, low-chromium steel plates (150 x 75 x 0.5 mm) were selected as the substrate material due to their propensity to oxidise. The plates were pretreated in a salt spray chamber to create a uniform and reproducible rust layer. Two application forms were tested – a liquid formulation and a gel formulation. The latter was thickened with xanthan gum TNAS-CS, an acid-stable, clear solution grade of xanthan gum. Both citric acid and phosphoric acid were tested at concentrations of 5%, 10%, and 20% (w/w).
Table 1: Test formulations with different concentrations of citric acid and phosphoric acid
| Active agent in subst. | Citric acid or phosphoric acid | Gel formulation (XG TNAS-CS) | Gel formulation (demineralised water) | Liquid formulation (demineralised water) | pH (citric acid) | pH (phosphoric acid) |
|---|---|---|---|---|---|---|
| 5% | 5g | 2.5g | 92.5g | 95.0g | 1.79 | 1.05 |
| 10% | 10g | 2.5g | 87.5g | 90.0g | 1.57 | 0.75 |
| 20% | 20g | 2.5g | 77.5g | 80.0g | 1.32 | 0.30 |
The gel was produced by preparing an aqueous citric acid solution into which xanthan gum was stirred at 800 rpm for two minutes. Preliminary tests had indicated that a minimum concentration of 5% citric acid is required to remove rust effectively. Concentrations below this threshold were insufficient. The liquid formulations were applied via 30-minute immersion, while the gel formulations were applied to the rusted surfaces for ten minutes. After treatment, the samples were rinsed and visually evaluated.
Both citric and phosphoric acid achieved satisfactory rust removal across all tested concentrations after 30 minutes immersion in a liquid soak solution (see figure 1). All tested concentrations of citric acid reliably removed the rust layer, suggesting that citric acid is indeed a viable alternative to phosphoric acid in this application. Moreover, qualitative differences in surface appearance were observed: after only 30 minutes phosphoric acid caused a brownish discolouration, indicating the onset of reoxidation. This effect was not observed with citric acid. Surfaces treated with citric acid appeared uniformly silver and shiny, suggesting better passivation or lower surface reactivity after treatment.

Figure 1: Results from performance testing of liquid formulation after 30 minutes of immersion and 30 minutes storage at ambient conditions
Results from tests on the gel products were comparable to those from the liquid formulations. Again, all concentrations of citric acid entirely removed the rust layer from the plates after ten minutes treatment time (see figure 2).
In conclusion, the results suggest that a concentration of 5–10% citric acid provides sufficient rust removing capacity in both liquid and gel formulations. Moreover, formulations that contain less than 10% of citric acid do not require hazard labelling according to current CLP Regulation (EC) No 1272/2008. For mixtures containing 10% citric acid or more a label with the hazard pictogram “warning” and the appropriate hazard statements may be required.
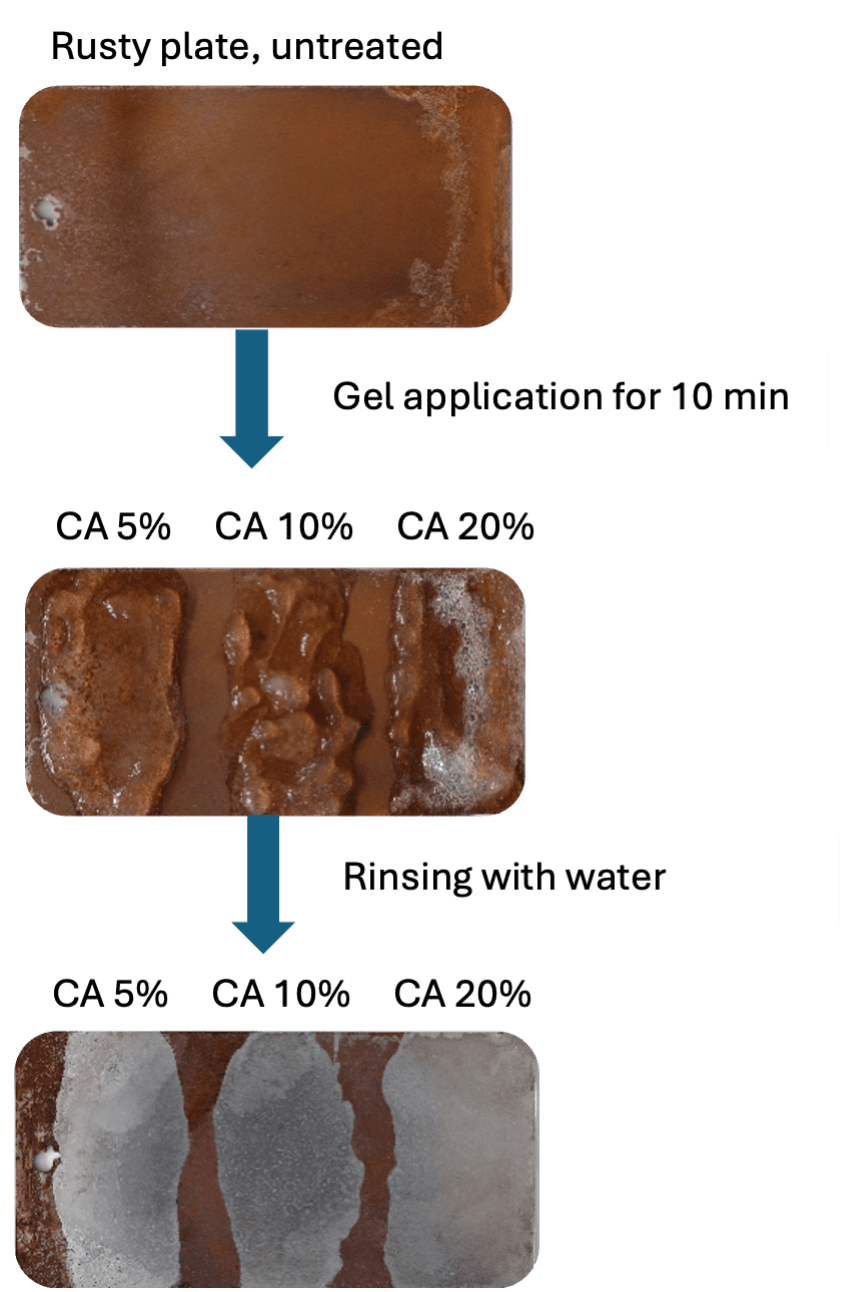
Figure 2: Results from performance testing of gel formulation containing different concentrations of citric acid (CA), after ten minutes treatment
In addition to the tests with phosphoric acid and citric acid described above, comparative tests were also conducted with industrial benchmark products containing phosphoric acid as the active ingredient. The performance of citric acid was equivalent to that of phosphoric-acid-based industry standard formulations, as shown in figure 3.
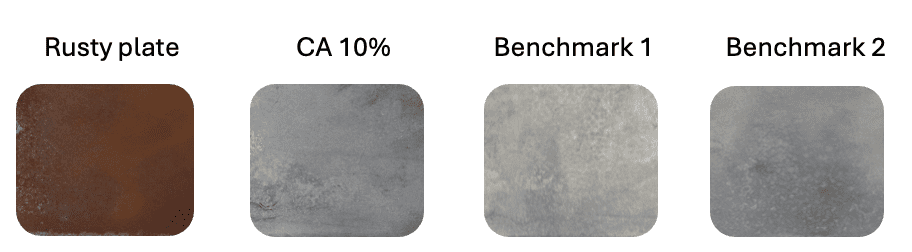
Figure 3: Results from performance testing of liquid formulation containing 10% citric acid (CA) after 30 minutes of immersion, compared to industry benchmark formulations with phosphoric acid as the active ingredient
The gel formulations containing xanthan gum and either citric or phosphoric acid were also tested for their stability. Viscosity was measured using an Anton Paar rheometer with plate geometry CP25. Both gel variants remained stable for two months at room temperature 25°C (77°F) and elevated temperature 40°C (104°F), without significant changes in viscosity (see figure 4). These findings confirm that the xanthan quality selected and the 2.5% concentration are suitable for formulating stable acidic gels and they underscore citric acid’s potential as an effective alternative to conventional phosphoric acid in rust removal applications.
Storage test gel formulation citric acid v. phosphoric acid
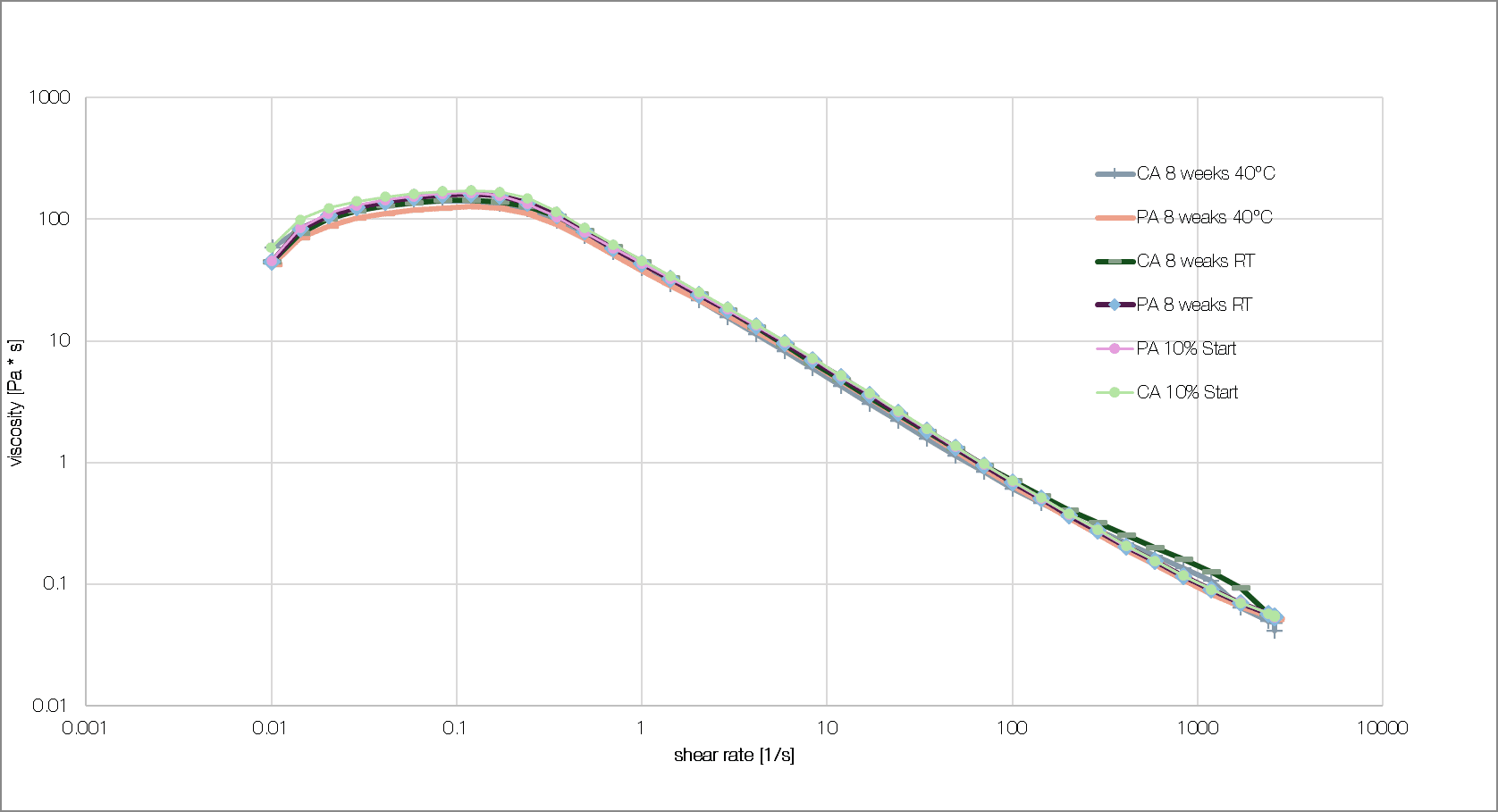
Figure 4: Gel storage test: viscosity was measured after eight weeks at room temperature (RT) and 40°C compared to the fresh formulation
Summary
This study demonstrates that the derusting performance of citric acid is comparable to that of phosphoric acid, while offering the additional benefit of reduced surface discolouration. The addition of acid-stable xanthan gum to the gel formulation ensured gel stability. Both formulations – liquid and gel – exhibited good application properties and stability, with the gel variant being particularly recommended for targeted, stationary applications.
Citric acid therefore represents a safe, biodegradable, and effective option for rust removal – especially appropriate for light to moderate corrosion, household applications, and sustainable product formulations. Its non-aggressive nature and low toxicity make it significantly safer to handle than mineral acids. Combined with its environmental compatibility and ability to preserve treated materials, it stands out as an attractive choice for modern, eco-conscious rust remover formulations.
The Authors
David Schäfer – Technical Support Specialist Acidulants, Jungbunzlauer International AG
Thomas von Känel – Product Manager Citrics, Jungbunzlauer International AG
Marianne Wohlfender – Strategic Marketing Manager Acidulants, Jungbunzlauer International AG
Katharina Wolf – Scientist, Jungbunzlauer Ladenburg GmbH
