Download this article as a PDF
Introduction
In recent years, direct compression has become increasingly prominent in tablet manufacturing, largely due to its efficiency in terms of labour, time, equipment needs, operational energy and space requirements. Direct compression offers the additional advantage of avoiding issues associated with heat and moisture that may arise during wet granulation. But direct compression places great demands on the ingredients used in the process. The active ingredients, and more especially the excipients, in tablet formulations must exhibit good compactibility as well as flowability.
Tricalcium Citrate TB (TCC TB) is a tricalcium citrate tetrahydrate produced using a unique process which creates the specific crystalline structure responsible for its outstanding characteristics – making it the ideal choice for a direct compression excipient.
Value proposition of TCC TB
– Excellent flowability due to specific crystal structure
– Compacts to tablets of higher tensile strength compared to other brittle fillers
– Enables production of hard tablets even at low compression pressures
– Low sensitivity to lubricant level ensures consistent compactibility
– Retains low relative density even when compressed to hard tablets
– Shows brittle fracture with plastic deformation and slight elastic relaxation
– Enables production of tablets with extremely flat surfaces and sharply defined edges
– Rapid disintegration due to high porosity
– Can be used for pharmaceutical products as well as for food supplements
– Organic calcium source in citrate form with excellent tolerability and compatibility
– Highest purity with low impurities and strict microbiological specification
Characteristics of TCC TB
TCC TB is Jungbunzlauer’s product name for tricalcium citrate tetrahydrate tableting grade and is specified to meet the requirements of the latest edition of the United States Pharmacopeia (USP), the Food Chemicals Codex (FCC) and Commission Regulation (EU) No 231/2012. TCC TB is halal and kosher certified, vegan, and allergen- and GMO-free.
Table 1 | Characteristics of TCC TB (typical values)
| IUPAC name | 2-hydroxy-1,2,3-propane-tricarboxylic acid calcium salt (2:3) | |
| Molecular formula | Ca3(C6H5O7)2 ·4H2O | |
| CAS No. | 5785-44-4 | |
| EC No. | 212-391-7 | |
| Appearance | White crystalline granular powder | |
| True density [g/cm3] | 1.89 | |
| Bulk density [g/L] | 500–600 | |
| Water activity [%] | 0.3 | |
| Water content [%] | 8.2 | |
| Water absorption [%]* | 0–21%rH | 4.6 |
| 21–98%rH | 1.0 | |
| Solubility [g/L] | 1.0 | |
| pH value (2.5% suspension) | 5.0–6.5 |
*and complete desorption
Flowability
The flowability of powders mainly depends on the particle size distribution and the shape of the particles. TCC TB exhibits good flowability, demonstrating robust behaviour as its flow is effectively independent of the applied shear rate. This outstanding performance is clearly reflected in the Hausner ratio, the compressibility index (Carr’s index), and the angle of repose (Table 2), and is further confirmed by shear cell analysis (Figure 1).
Table 2 | Flowability characterisation of TCC TB (typical values)
| TCC TB | Thresholds indicating “good flowability”* | ||
| Hausner ratio [-] | 1.16 | 1.12–1.18 | |
| Compressibility index (Carr’s index) [-] | 13.8 | 11–15 | |
| Angle of repose [º] | 29 | 31–35 | |
*according to European Pharmacopoeia (Ph. Eur.) method 2.9.36. Powder flow.
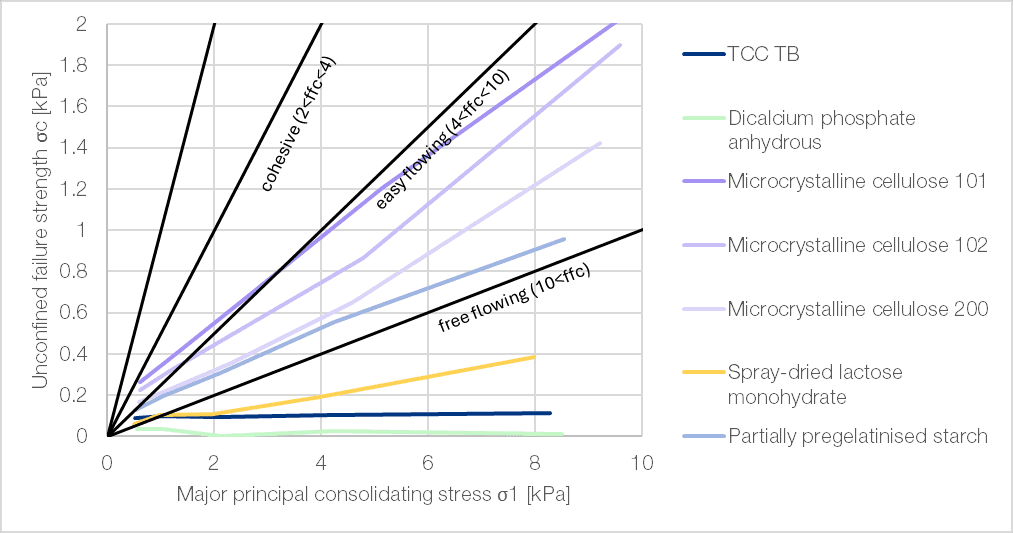
Figure 1 | Flow behaviour under consolidated conditions as measured by shear strength
Powder permeability
Air permeability is a material property that plays a critical role during die filling in the tableting process. Together with flowability, it contributes to the consistency and uniformity of tablet mass, while reducing the risk of entrapment-related defects during compaction. This property is particularly important in the manufacture of multilayer tablets, where gravity filling is employed and high permeability is beneficial. TCC TB not only exhibits good flow behaviour but also demonstrates favourable high air permeability (Figure 2). As a result, it is possible to achieve low mass variation and minimal tablet defects during tablet production.
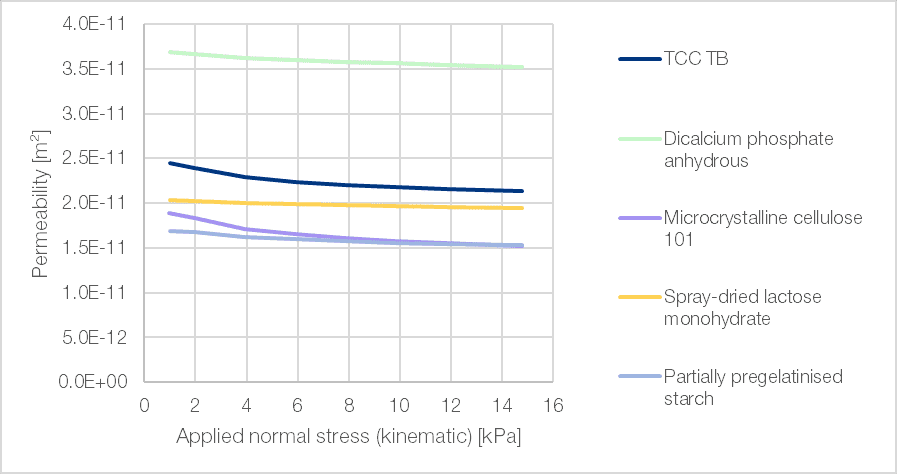
Figure 2 | Permeability of powders measured under normal stresses between 1 and 15 kPa with an air velocity of 20 mm/s
Compactibility
TCC TB enables the production of hard tablets using reasonable compression forces. Within the range of brittle fracture excipients tested, TCC TB stood out in its ability to produce tablets of sufficient tensile strength even at relatively low compaction pressures, thus reducing wear on tableting equipment (Figure 3).
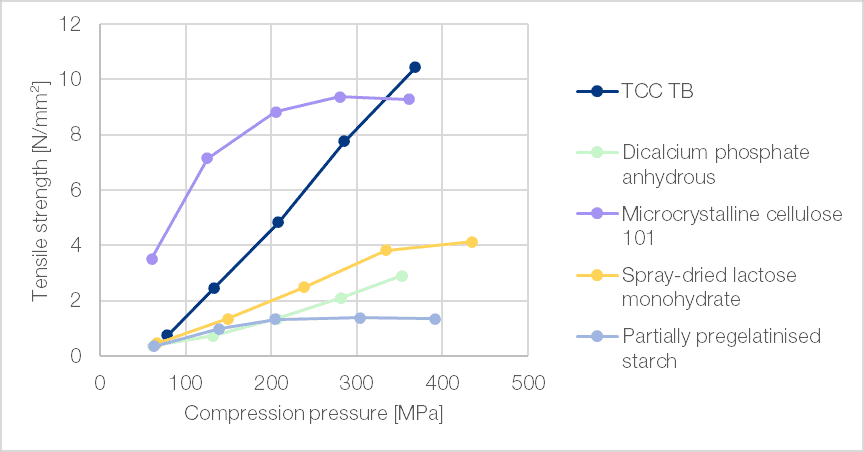
Figure 3 | Compactibility plot (StylOne Evolution, 9 mm concave (r = 15 mm) mimicking a Fette 1200 at low speed (17 rpm))[1]
The production of tablets with TCC TB is independent of compression speed, with tablets produced at high compression speed exhibiting comparable hardness to those produced at medium or low compression speed (Figure 4). This speed insensitivity of TCC TB is an important advantage, as it prevents problems from occurring during up-scaling due to increased punch velocity when transferring the product from a station laboratory press to a rotary press.
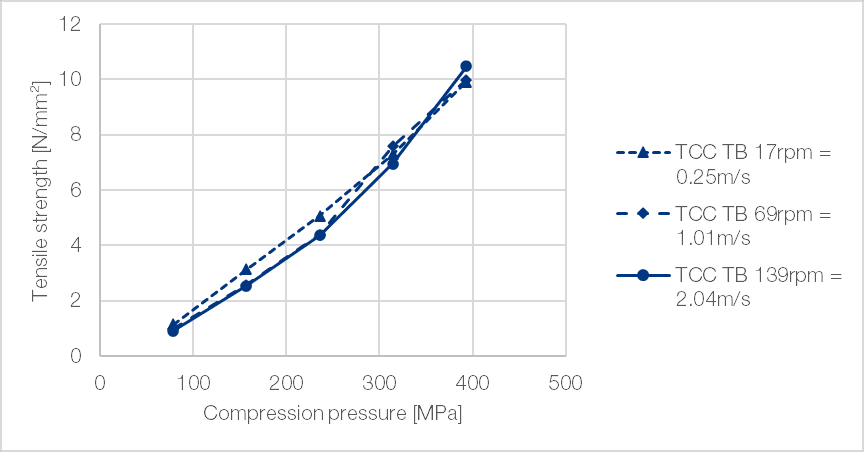
Figure 4 | Independency of TCC TB from compression speed. Compactibility plot at high compression speed (139 rpm=2.04 m/s), medium (69 rpm=1.01 m/s) and low speed (17 rpm=0.25 m/s) on a rotary tablet press (Fette 102i; 9 mm concave (r = 15 mm))[1]
The compression trials on the rotary press showed that the compressibility of TCC TB (+0.5% magnesium stearate as lubricant) is independent whether or not pre-compression is used.
Compression behaviour
At the onset of compression, TCC TB undergoes an initial phase of particle rearrangement. Afterwards the particles show brittle fracture with plastic and only slight elastic deformation (Figure 5). Compared to other brittle substances like dicalcium phosphate anhydrous or spray-dried lactose, the elastic relaxation of TCC TB is slightly more pronounced (Table 3).
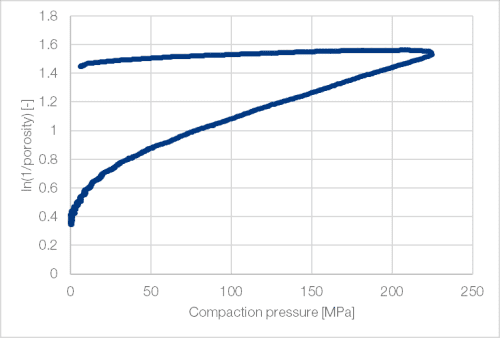
Figure 5: Heckel plot of TCC TB (StylOne Classic 105 ML; 8 mm flat)[1]
| Elastic relaxation [%] | |
| TCC TB | 5.3 |
| Dicalcium phosphate anhydrous | 5.5 |
| Microcrystalline cellulose 101 | 7.9 |
| Spray-dried lactose monohydrate | 3.7 |
| Partially pregelatinized starch | 21.3 |
Table 3 | Elastic relaxation, in- and out-of-die method 24 h[1]
With its relatively low elastic relaxation, TCC TB enables a subsequent coating step without a long resting period for post-compaction viscoelastic strain recovery. At the same time, it retains sufficient relaxation to avoid tablet defects when combining it with other, more elastic, tablet constituents.
Porosity retention
When compressed, TCC TB produce tablets of high strength even with low compression forces. These tablets tend to retain their low relative density compared to other excipients in common use (Figure 6). This higher porosity facilitates the disintegration and dissolution of the tablets.
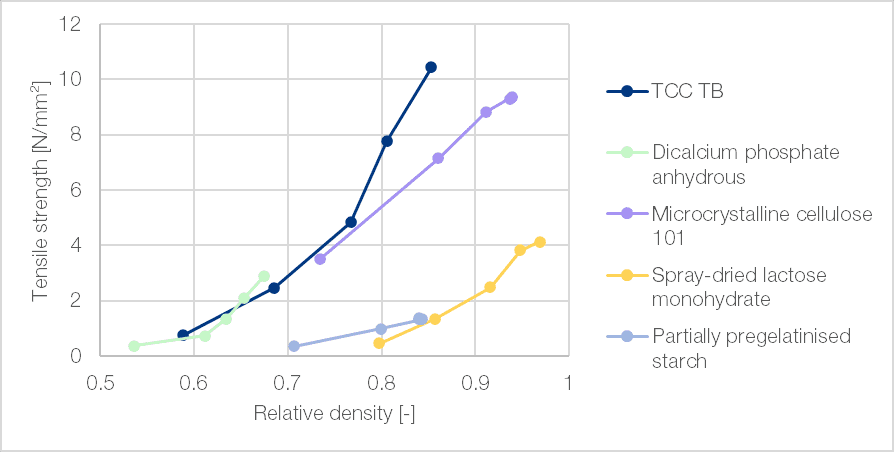
Figure 6 | Bondability plot (StylOne Evolution, 9 mm concave (r = 15 mm) mimicking a Fette 1200 at low speed (17 rpm))[1]
Disintegration
Tablets produced from TCC TB disintegrated easily even without the use of disintegrants, with disintegration times below 3 min at tensile strengths of up to 8 N/mm². These figures were reproducible and were not affected by whether or not pre-compression was applied. Figure 7 shows data for tablets produced with pre-compression only. Note that with a tensile strength of 10 N/mm² there was an exponential increase in disintegration time.
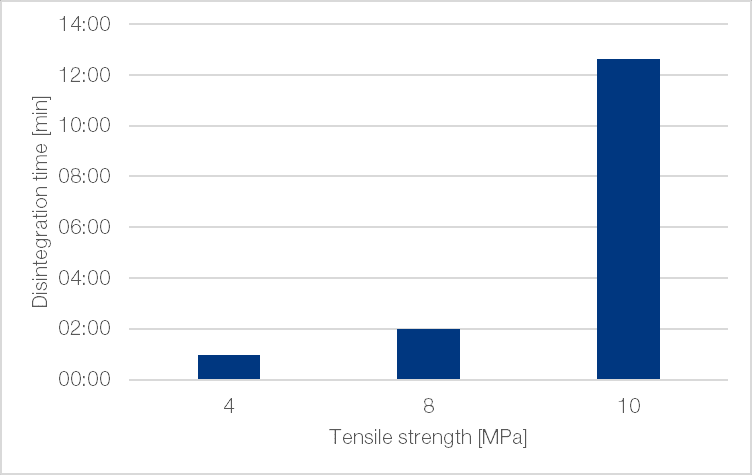
Figure 7 | Disintegration time of TCC TB tablets with 0.5% magnesium stearate as internal lubricant (Fette 102i, 9 mm round concave (r = 15 mm); with pre-compression; 69 rpm (1.01 m/s))
Case study: loading capacity
The influence of an active ingredient on processability, including parameters such as powder flow and tabletability, is of significant importance. Typically, the more active ingredient a formulation contains, the more its properties differ from those of pure excipient. Hence direct compression tableting is challenging. Figure 8 illustrates the loading capacity of TCC TB, exemplified using two different substances – fine crystalline paracetamol powder and hygroscopic ginseng extract – both of which are typically required in high doses in tablet formulations and are known to have a poor compaction behaviour.
Mixtures of TCC TB and fine paracetamol powder at drug loads between 20% and 40% and mixtures containing 20% to 30% ginseng extract were compacted on a single punch tablet press. Tablets produced with TCC TB showed high tensile strength (>1.5 N/mm²) for all drug loads tested.
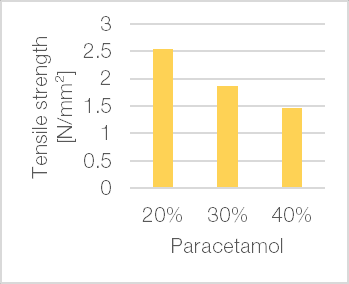
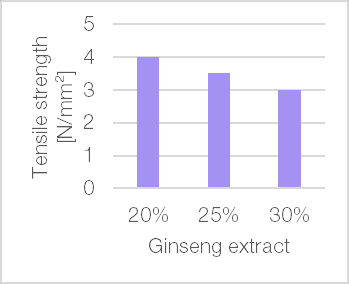
Figure 8 | Mechanical strength of mixtures of TCC TB and (a) fine paracetamol powder with 1.0% magnesium stearate as internal lubricant (StylOne Classic 105 ML; 8 mm flat; approximately 250 MPa compression pressure; at 25% speed: 225 tablets/h); (b) ginseng extract with 0.5% magnesium stearate as internal lubricant and 0.5% Aerosil® as flow aid (StylOne Classic 105 ML mimicking a Fette 102i, 8 EuB; 10 mm flat-face tooling; running at 67 rpm (0.98 m/s) at approximately 200 MPa compression pressure with pre-compression pressure of 20%)
Dissolution of these paracetamol tablets was very fast (Apparatus 2 (paddle), 75 rpm, medium: 900 ml 0.1 N HCl, 37 ± 0.5°C.). All formulations (20%, 30% and 40% paracetamol load) released more than 83% paracetamol after 2 min. Similarly, in formulations containing ginseng extract, TCC TB consistently resulted in shorter disintegration times at identical disintegrant levels compared to alternative fillers. This suggests improved tablet disintegration and a potential advantage in terms of dissolution performance.
Case study: moisture sensitive active ingredient
Depending on the formulation used, drug-excipient interactions were found to adversely affect the stability of the product. It was shown that a condensation layer forming on the surface of the excipient is responsible for the degradation of enalapril maleate into enalaprilat or diketopiperazine (Figure 9). Consequently, the prodrug function of enalapril is lost. Furthermore, diketopiperazine exhibits no therapeutic activity, representing a direct loss of active pharmaceutical ingredient.[2]
When TCC TB was used as the excipient in a formulation containing 6% enalapril maleate as active ingredient and 0.5% magnesium stearate as an internal lubricant, no issues were observed with respect to flow behaviour, tabletability, friability, or disintegration time. Regarding the stability of enalapril maleate, TCC TB demonstrated superior performance compared to a co-processed excipient of spray-dried lactose and starch, a combination of two fillers found in a commercially available enalapril product. The stability of the TCC TB-based formulation could be further enhanced by the addition of 5% trimagnesium citrate anhydrous (TMC), which is attributed to its desiccant properties.
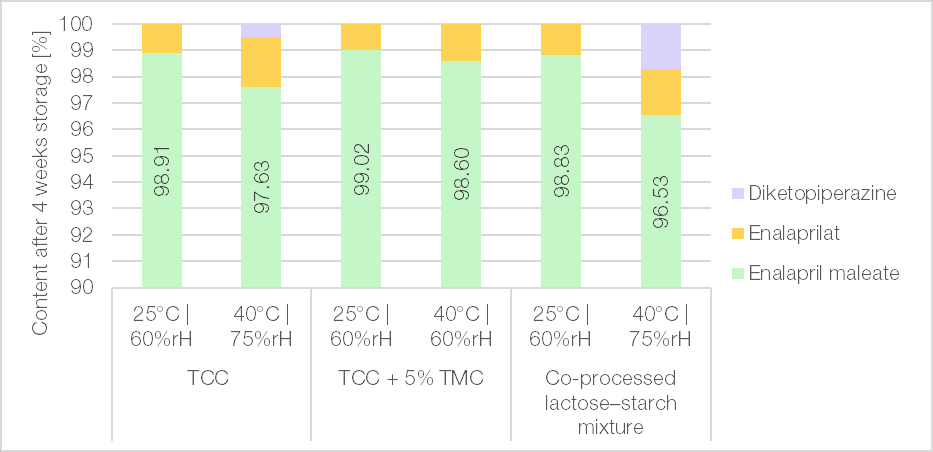
Figure 9 | Four-week stability of enalapril tablets with 6% enalapril maleate and 1% magnesium stearate in closed containers at standard (25°C/60%rH) and accelerated (40°C/75%rH) conditions (Fette 102i; 7 mm concave (r = 10 mm); tensile strength of 1.5 N/mm²). The remaining content of enalapril maleate and its degradation products are shown.
Case study: minitablets
Minitablets with a diameter of 2.0 mm open up novel therapeutic options for patients experiencing swallowing difficulties. They are particularly well suited for paediatric and geriatric populations, where flexible and individualised dosing is often required, as they are both easy to adapt and to swallow.
In this case study, formulations containing TCC TB plus 12.5% furosemide and 1% magnesium stearate were used, with tablet masses of approximately 8 mg. These yielded a dosage of 1 mg per minitablet. In relation to the standard dosage of the model drug furosemide (1 mg/kg body weight, maximum dosage 40 mg), the resulting calcium intake corresponds to a maximum of 5.5% of the dietary reference values for children.[3] This means that there should be no significant impact on overall calcium intake.
Unlike many minitablet formulations, which require a granulation step to achieve suitable flow and compressibility, TCC TB enables direct compression. This eliminates the need for granulation, streamlining the manufacturing process and reducing complexity.
Although the addition of furosemide significantly impaired the otherwise excellent flow properties of the formulation, acceptable flow behaviour was still achieved. Content uniformity, which is critical for minitablet production, was maintained, and TCC TB provided sufficient tensile strength (>1 N/mm²). The tableting process proceeded smoothly, and as previously observed with TCC TB, disintegration times remained very short (<10 s).[4]

References
[1] Hagelstein V, Gerhart M, Wagner KG. Tricalcium citrate – a new brittle tableting excipient for direct compression and dry granulation with enormous hardness yield. Drug Dev Ind Pharm 2018;44(10):1631–1641.
[2] Al-Omari M, Abdelah MK, Badwan AA, Jaber AMY. Effect of the drug-matrix on the stability of enalapril maleate in tablet formulations. J Pharm Biomed Anal 2001;25(5-6):893–902.
[3] EFSA Panel on Dietetic Products NaA. Scientific opinion on dietary reference values for calcium. EFSA Journal 2015;13(5): Article 4101.
[4] Hafels M, Mattusch A, Steffens K, Wagner KG. Tricalcium citrate TB as effective filler in direct compression of minitablets. 5th European Conference on Pharmaceutics, Porto, Portugal, 2025
Parts of the data presented in this study were generated at the Department of Pharmaceutical Technology and Biopharmaceutics of the University of Bonn.
About Jungbunzlauer
Jungbunzlauer is a leading producer of high-quality, sustainable ingredients from natural sources, serving industries from food and beverage, to nutrition, health, home and personal care, among others. Leading the way in developing naturally better ingredients that enhance everyday life, we are a trusted partner offering a diverse portfolio of texturants, acidulants, sweeteners, minerals, and tailored solutions to meet our customers’ evolving needs.
Headquartered in Basel, Switzerland, with state-of-the-art facilities including large-scale fermentation operations across Europe and North America, we proudly serve more than 130 countries worldwide. Founded more than 150 years ago, Jungbunzlauer has grown into a CHF 1.3 billion company, driven by nearly 1,400 dedicated colleagues committed to a healthier, more sustainable future.
The authors
Markus Gerhart – Senior Director Category Lead Minerals & Solutions, Jungbunzlauer Ladenburg GmbH
Amelie Mattusch – Senior Scientist Application Development Particle Technology, Jungbunzlauer Ladenburg GmbH
Thomas Eckert – Senior Product Manager Minerals in Product Group Management, Jungbunzlauer Ladenburg GmbH
The information contained herein has been compiled carefully to the best of our knowledge. We do not accept any responsibility or liability for the information given in respect to the described product. Full and sole responsibility for use of our products lies with the user, especially in respect to third-party patent rights and to laws or government regulations.
©2025 087-25 v1 Jungbunzlauer Suisse AG
